Introduction
Automatic
biochemical analyzer is a kind of instrument that imitates manual operation to
complete some or all of the steps in biochemical analysis, such as sampling,
reagent addition, removing interferences, mixing, heat preservation,
colorimetry, result calculation, writing report and cleaning. It can be used for the analysis and determination of various reaction
types such as timing method and continuous monitoring method.
Besides the determination of general biochemical items, some can also be
used to measure the hormone, immunoglobulin, blood concentration and other
special compounds, as well as the application of enzyme immunity and
fluorescence immunoassay.

Automatic biochemical
analyzer
Basic principles
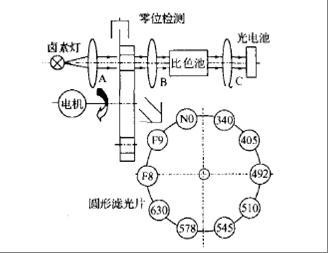
Automatic
biochemical analyzer belongs to optical analytical instrument, which is based
on the selective absorption of light by substances, namely spectrophotometry.
Monochromator divides the complex light from light source into monochromatic
light. Then, the monochromatic light of a specific wavelength passes through
the colorimetric cell containing the sample solution, and the photoelectric
converter converts the transmitted light into electrical signal, which is sent
to the signal processing system for analysis.
Equation
Spectrophotometer is a method based on the selective absorption of
electromagnetic radiation by different molecular structures, which belongs to
the analysis of molecular absorption spectrum. When
the light passes through the solution, the molecule of the measured substance
absorbs the monochromatic light of a certain wavelength, and the intensity of
the absorbed light is proportional to the distance of the light passing
through.
Although
it is known that Bouguer proposed the mathematical expression of the above
relationship as early as 1729, it is generally believed that Lambert first
found the expression in 1760.
The
mathematical formula is as follows:
T=I/I0=e-kb
Where I0 is the intensity of incident light, I is the intensity of transmitted light, e is the base of natural logarithm, k is the constant, b is the length of Optical path (commonly expressed in cm).
Beer’s
law is equivalent to Bouguer's law, but Beer's law is expressed in terms of
concentration. The two laws are combined to form the Beer Bouguer law:
T=I/I0=e-kbc
Where C is the concentration of the light
absorbing substance (common unit in g /
L or mg / L). The linear
expression is obtained by taking the logarithm of 10 as the base:
A=-logT=-log(I/I0)=log(I0/I)=εbc
Where A is the absorbance, ε Is the molar absorption coefficient
or extinction coefficient.
The
above expression is usually called beer's law. It
shows that when the monochromatic light of a certain wavelength passes through
the solution, the absorbance of the sample is directly proportional to the
concentration of the absorber in the solution and the distance of the light
passing through.
Common devices
Emitter:
Bulb 64258, LED, etc.
Receiver:
Single-Transistor:PC10-2,PC10-6,PIN-13DSB,PIN-6.6DPC
There
are 8 channels on one instrument, which represent 8 detection wavelengths.
Eight photocells are needed respectively.
Arrays:A2C-16-1.57-SB,A5V-35UV
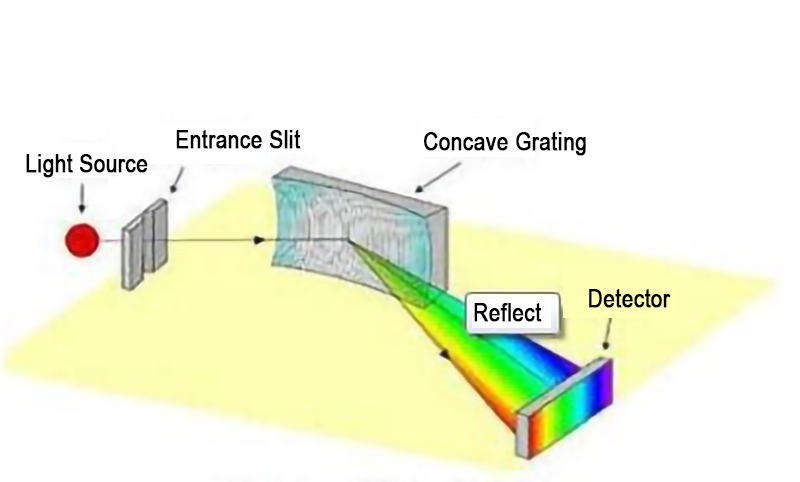
Optical path
RM 707-708, 258 Guoxia Road, Yangpu District,Shanghai, China PC:200433
Copyright © 2019-2020.Light-Catcher Co.,Ltd All rights reserved.沪ICP备15002270号-1

